Demonstrated 15% reduced risk in time to CV death, heart attack, stroke, hospitalization for unstable angina or coronary revascularization, whichever occurred first vs. placebo ( HR 0.85, 95% CI: 0.79, 0.92; p < 0.0001; patients with event: Repatha® 9.75%, placebo 11.34%).

FOURIER Cardiovascular Outcomes Trial1,4:
Repatha® + statin (key secondary endpoint)
Demonstrated significant risk reduction in time to MI, stroke, or CV death, whichever occurred first vs. placebo + statin
Time to CV death was not statistically significant vs. placebo (p=0.6188)
Demonstrated 20% RRR in time to CV death, heart attack, or stroke HR 0.80 (95% CI: 0.73, 0.88; p < 0.0001; patients with event: Repatha® 5.92%, placebo 7.35%)
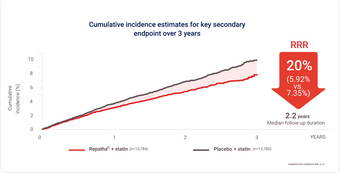
Primary composite endpoint
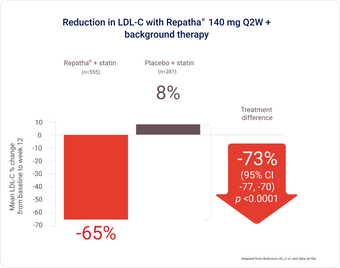
LAPLACE-2 study: Powerful LDL-C reduction shown in patients with primary hyperlipidemia1,5
Overall study population included those with ASCVD
Repatha® Q2W + statin provided an additional 73% LDL-C reduction overall (vs. placebo + statin; p < 0.0001).*
Prevention of Cardiovascular Events: Repatha® is indicated as an adjunct to diet and standard of care therapy (including moderate- to high-intensity statin therapy alone or in combination with other lipid-lowering therapy), to reduce the risk of myocardial infarction, stroke, and coronary revascularization in adult patients with atherosclerotic cardiovascular disease (ASCVD) by further lowering low-density lipoprotein cholesterol (LDL-C) levels.
Primary Hyperlipidemia (including Heterozygous Familial Hypercholesterolemia [HeFH] and ASCVD): Repatha® is indicated for the reduction of elevated low density lipoprotein cholesterol (LDL-C) in adult patients with primary hyperlipidemia (including heterozygous familial hypercholesterolemia and ASCVD): as an adjunct to diet and statin therapy, with or without other lipid-lowering therapies, in patients who require additional lowering of LDL-C; as an adjunct to diet, alone or in combination with non-statin lipid-lowering therapies, in patients for whom a statin is contraindicated.
Up to 95% of patients achieved LDL-C < 1.8 mmol/L with Repatha® (Q2W and QM doses).
* Overall difference, mean % change from baseline to week 12 in LDL-C, mean difference from placebo with Repatha® 140 mg Q2W (95% CI: -77%, -70%; p < 0.0001).
RUTHERFORD-2 study: Powerful LDL-C reduction in high-risk patients with HeFH1,6
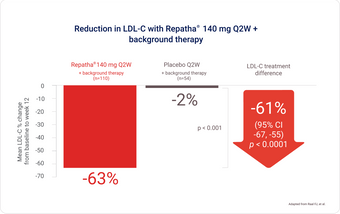
Background therapy: statin ± other lipid-lowering therapy, at 12 weeks.
Repatha® provided 61% additional reduction in mean LDL-C vs. patients on background therapy alone.

